Prashant Jain
Energy from Light Stored, Shuttled, and Used on Demand
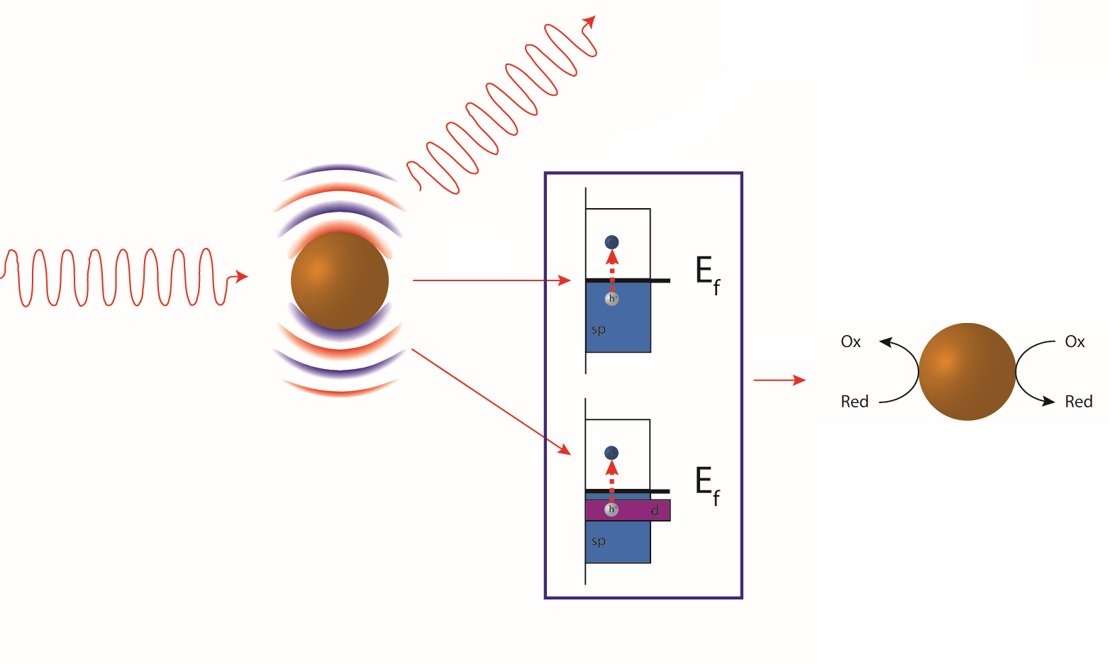
The intermittency of solar radiation is a major bottleneck in the widespread adoption of photovoltaics for renewable power generation. Being able to store energy harvested from the sun and deploying this stored energy on demand is a central challenge of our times. Nature has solved this challenge: photosynthetic organisms harvest solar energy and store it in the form of redox cofactors. Taking a page from nature, Professor Jain envisages a new solution to this challenge where light will be harvested by specially designed nanoscale antenna and stored in high density in the form of long-lived energetic chemical species. Upon demand, the energy carriers will be transported to a manufacturing site where they will be used to power thermodynamically uphill reactions for the green production of chemicals and fuels. In this manner, sunlight can be deployed on demand as a source of energy, a chemical reagent, and a tool for building complex molecules. For harvesting solar radiation, Professor Jain will engineer nanoscale architectures optimized to function as antennas for capturing light and converting it to energetic charge carriers.
Metal complexes with multiple oxidation states will be used to capture these energetic carriers and stabilize them so that they can be stored and transported to the manufacturing site. As one manufacturing goal, the energy carriers will be used to power the conversion of nitrogen gas to ammonia, a high-demand chemical required for food production and a hydrogen energy economy. The conversion of nitrogen gas to ammonia is a demanding reaction carried out on a global scale with immense energy demands; the conventional Haber-Bosch process uses an estimated 1% of the world's energy consumption. This new strategy will lead to the sustainable method for local ammonia production using abundant renewable resources such as nitrogen gas, water, and sunlight without the need for fossil-fuel-derived hydrogen gas.
